
I’m sure I am not alone in having felt somewhat skeptical about ever reaching an energy utopia. As the world scrambles to extract every last drop of oil and whiff of natural gas, realistic solutions seem few and far between.
But my view changed last year when, in a slightly stuffy tent at the Cheltenham Science Festival, a curly-haired biophysicist from Cambridge clambered up onto the stage. As Chris Forman spoke about looking to nature to inspire technology, he made me think about energy in a radically different way.
If we could harness, store, and use energy as thriftily as natural systems do, there’s potential to solve many of our power generation problems in the blink of an eye. Forman believes that by copying and improving upon natural systems, we could do exactly that. While this might sound far-fetched, much of the research necessary to make Forman’s energy utopia a reality is already underway.
Let’s start at the beginning. While harnessing energy may be the weakest link in our chain, nature has evolved an extremely efficient system for generating it–photosynthesis.
Maybe you remember how it works from school: When sunlight hits the leaves of a plant, electrons are transferred from water to special “carrier” molecules, before being used to produce sugars for storage in the plant’s cells. “Nature is extremely good at converting light energy into fuel,” said Dr. Lars Jeuken, a synthetic biologist at the University of Leeds. Plants minimize energy loss by moving electrons around very quickly, though they use most of the energy they capture just to stay alive–only 1 to 8 percent ends up as plant tissue.

Yet almost all our energy comes from photosynthesis, albeit rather indirectly. Fossil fuels are formed from the crushed remains of ancient plants and the animals that ate them. Which, when you think about it, is a pretty convoluted way of harnessing solar energy. Worse still, we waste more than 70 percent of the energy created from them at the power plant.
Human technology already surpasses the sun-harnessing capabilities of plants–the best commercially available solar panels capture 15 percent of the light energy that hits them, and some experimental designs are reaching up to 40 percent. But synthetic biologists think they can do better–by engineering nature, hoping to beat it at its own game.
Dr. Erwin Reisner supervises a team of researchers at the University of Cambridge working on artificial photosynthetic systems to produce hydrogen fuel. “One aim of my laboratory is the development of efficient solar fuel synthesis by combining biological and synthetic approaches,” said Reisner. His team uses a synthetic dye to capture light energy, which is then converted into hydrogen fuel by biological catalysts called enzymes. Together, the enzymes and synthetic dyes are integrated into a nanostructured photoactive material–an artificial leaf, of sorts. While hybrid systems like this could one day surpass solar panels, Reisner stresses that this is a long-term project. “I hope this research will improve the basic understanding of artificial photosynthesis, to enable the large-scale implementation of solar fuel technologies in the next few decades,” he said.
Jeuken is part of a collaborative project, with Reisner and researchers at the University of East Anglia in England, to improve the efficiency of electron transfer in artificial photosynthetic systems. “The latest hybrid systems are very far from where we want them to be,” he said–as they convert just 2 percent of light energy into fuel. The key to improving efficiency, to equal or even surpass natural photosynthesis, will be to speed up the reactions, he told me–the faster we move the electrons, the less energy is wasted.
But artificial leaves only generate energy when the sun shines. Innovative new ways to store energy will be essential as we come to rely on the intermittent supply from renewable sources. Again, our best bet may be to try and imitate nature and, ultimately, improve upon it.
Professor Percival Zhang at Virginia Tech has developed a battery that mimics energy storage in living cells. Nature stores energy in the form of sugars–these carbohydrate molecules are stable, easily recycled, and their production is carefully controlled by enzymes, making them highly efficient storage molecules. Enzymes control every chemical reaction inside the cell, and they are a synthetic biologist’s dream–extremely flexible and easily reprogrammable.
Zhang’s sugar-based biobatteries use 13 synthetic enzymes to produce electricity from the sugar maltodextrin. Synthetic enzymes allow for extremely efficient energy release, and the biobatteries have a host of other benefits, too–sugar is a cheap, renewable energy source; it is also biodegradable and totally safe.
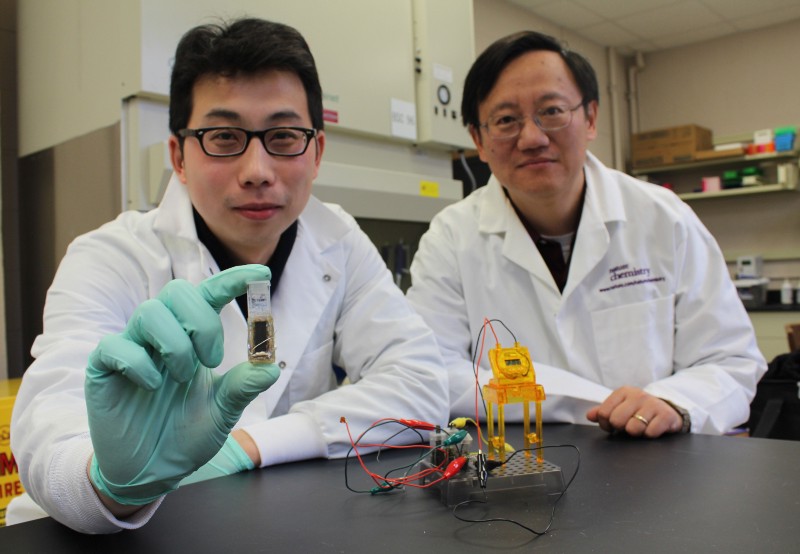
But there is still work to be done. Sugar can store more energy than traditional batteries, but the technology currently has a low power output per square inch, meaning sugar batteries would need to be huge to power our energy-hungry tech. “We believe we can increase its power density ten-fold in the next several years,” said Zhang. By fine-tuning the technology with “better nanomaterials, better enzymes, and better fuel cell design,” they hope to develop batteries that will outperform natural energy storage systems evolved over millions of years.
Another challenge is that enzymes slowly degrade, giving the biobattery a short shelf life. At present, it can run for just a few weeks, but Zhang hopes to extend that to months, even years, by engineering more stable enzymes. He aims to have the first sugar batteries on the market within three years and is already building prototype power banks for charging cell phones.

However we produce it, we need a way to transport energy to those places it is needed. Rather than technological innovation, this stage of the process might benefit from a change in perspective. For his part, Forman believes that locally produced electricity will play a much larger role in the future.
“Roughly speaking, the more energy stored in a material, the more efficient it is to move that energy over long distances,” he explained. But “renewable energy sources generally provide a lower power output,” so “we won’t be moving electricity as far as we have in the past.”
We would be better suited by investing in small-scale, localized generation at sites where energy is actually needed–and better batteries, Forman recommends. Right now, that might mean solar panels on the roof and a Tesla battery in your attic, but perhaps one day every building will have a garden of artificial plants plugged into a sugar battery.
We can also make better use of the solar energy we harness. For example, Wissington Sugar Factory in Norfolk, England, ferments its waste beets to produce the energy required to drive the beet-processing machines. This innovation doesn’t make use of nanotechnology or synthetic biology; its breakthrough was changing the perspective around energy and waste. Meanwhile, Forman and his collaborators have a few ideas for how synthetic biology might take the idea further. By engineering the plants or microbes we grow, we could produce virtually any chemical we wanted. “The same local factory could produce electrical energy, biofuels, construction materials, and food–as well as taking care of the recycling!” he said.

Zhang and his team also have big dreams for sugar within our future energy supply. Although many people, including myself, have argued against replacing fossil fuels with biofuels because they compete with crops for agricultural land, perhaps this argument misses the point entirely. Zhang’s batteries remind us that food and fuel are one and the same.
Zhang’s energy utopia involves a three-fuel system of electricity, sugar, and hydrogen–which he suggests is our best solution for long-term sustainable development. Electricity is a universal fuel, and hydrogen is an extremely clean storage compound, he noted. The ideal intermediate is carbohydrate, since it also doubles as a source of food. This energy utopia could be produced using a combination of artificial photosynthesis and biobatteries–imagine the global impact if food and fuel became completely interchangeable.
Combining innovative new nanotechnology, the flexibility of synthetic biology, and intelligent infrastructure could be our fast track to an energy utopia. If we can engineer new and improved leaves to produce renewable fuels convertible into electricity or fuel, we could solve the energy crisis–and maybe even feed the world along the way.


How We Get To Next was a magazine that explored the future of science, technology, and culture from 2014 to 2019. This article is part of our Power Up section, which looks at the future of electricity and energy. Click the logo to read more.
